
Natto Extract Powder, Nattokinase 20000FU/G
Nattokinase is a kind of enzyme extracted and isolated from natto and used traditionally for heart conditions to relieve fatigue, and as an anti-beriberi agent. And the available enzyme bioactivity we have include 50,000 FU/G(UV); 20,000 FU/G(UV); 5,000 FU/G(UV).
Nattokinase/Natto Extract
【Names】: Natto Extract, BSP, Extrait de Natto, Fermented Soybeans, Haricots de Soja Fermentés, Natto de Soja, Nattokinasa, Nattokinase, Soy Natto, Subtilisin NAT, NK.
【Botanical Source: Phaseolus vulgaris
【Part Used】: Seed
【Specification】: 5000FU/G,10,000FU/G, 15,000FU/G, 20,000FU/G,30,000FU/G, 40,000FU/G,50,000FU/G
【Fermentative Strain】: Bacillus natto
【Specification】: 50,000 FU/G(UV); 20,000 FU/G(UV); 5,000 FU/G(UV); Natto freeze-dried powder
【Packing】: 1kg, 5kg/bag(LDPE inside and aluminum foil bag outside; Vacuum-packed); 10kg, 25kg/aluminum drum
 Nattokinase is a kind of enzyme extracted and isolated from a traditional Japanese food named natto. Natto is made from soybeans fermented with Bacillus subtilis var. natto. and is a traditional Japanese food consumed for at least a thousand years as breakfast with rice, on toast, or as sushi, and is also available as an ice cream flavor. Natto is produced by fermentation by adding the bacterium Bacillus natto to boiled soybeans. Nattokinase is not related to any of the known kinases but is a serine protease produced by the bacterium acting on the soybeans. While other soy foods contain enzymes, it is only the natto preparation that contains the specific nattokinase enzyme. Nattokinase is named for the fact that it is an enzyme produced by nattokin, the Japanese name for Bacillus subtilis var natto. The Japanese researcher Hiroyuki Sum at the Chicago University Medical School firstly found that natto can dissolve artificial fibrin in 1980. Sumi and his team separated an novel enzyme from natto which they named as “nattokinase” , and they found that nattokinase can degrade fibrin as well as plasmin substrate. Nattokinase has been extensively studied in Japan, Korea, and China and used traditionally for heart conditions to relieve fatigue, and as an anti-beriberi agent. In recent years, the Western medicine has gradually recognized the anti-clotting effect of nattokinase. The safety of nattokinase has been studied and evaluated by the National Sinence Foundation in the United States and the clinical trial study has come to stage on its atherothrombotic prevention effect in the USA.
Nattokinase is a kind of enzyme extracted and isolated from a traditional Japanese food named natto. Natto is made from soybeans fermented with Bacillus subtilis var. natto. and is a traditional Japanese food consumed for at least a thousand years as breakfast with rice, on toast, or as sushi, and is also available as an ice cream flavor. Natto is produced by fermentation by adding the bacterium Bacillus natto to boiled soybeans. Nattokinase is not related to any of the known kinases but is a serine protease produced by the bacterium acting on the soybeans. While other soy foods contain enzymes, it is only the natto preparation that contains the specific nattokinase enzyme. Nattokinase is named for the fact that it is an enzyme produced by nattokin, the Japanese name for Bacillus subtilis var natto. The Japanese researcher Hiroyuki Sum at the Chicago University Medical School firstly found that natto can dissolve artificial fibrin in 1980. Sumi and his team separated an novel enzyme from natto which they named as “nattokinase” , and they found that nattokinase can degrade fibrin as well as plasmin substrate. Nattokinase has been extensively studied in Japan, Korea, and China and used traditionally for heart conditions to relieve fatigue, and as an anti-beriberi agent. In recent years, the Western medicine has gradually recognized the anti-clotting effect of nattokinase. The safety of nattokinase has been studied and evaluated by the National Sinence Foundation in the United States and the clinical trial study has come to stage on its atherothrombotic prevention effect in the USA.
Traditional source of nattokinase is mainly extracted from the bacterium Bacillus subtilis fermented soybeans. However this source usually contains the Vitamin K2, soy isoflavone, soy protein tissue and purine which may raise the risk of apoplexia. More importantly, some soybean is related to the GMO material. To eliminate these bad factors, we work closely with several universities and research institutes to independently develop a new source of nattokinase from kidney beans. White kidney bean, red kidney bean, black kidney bean are used to mass produce the nattokinase by fermenting with the bacterium Baccillus Subtilis instead of the traditional soybean. And the available enzyme bioactivity we have include 50,000 FU/G(UV); 20,000 FU/G(UV); 5,000 FU/G(UV).
How does Nattokinase work?
Through dissolving cross-liNattokinaseed fibrin and plasmin substrate, converting internal prourokinase to urokinase, inactivating plasminogen activator inhibitor-1, increasing the release of tissue plasminogen activator (t-PA) that supports fibrinolytic activity and inhibiting platelet aggregation by blocking thromboxane, nattokinase can help to break down the blood clots effectively. Meanwhile, nattokinase bears very little to no side effects compared with regular fibrinolytic proteases such as tissue plasminogen activator and urokinase which may cause bleeding. Orally taken of nattokinase can be absorbed by the intestinal tract in Fujita’ study(Transport of nattokinase across the rat intestinal tract. Fujita M, Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, Nishimuro S, Biol Pharm Bull. 1995 Sep; 18(9):1194-6.) and nattokinase was proved to have fibrinolytic effect after absorbed in duodenum. By thinning the blood clot, nattokinase might decrease the risks of stroke, heart attack, and other conditions that relating to blood clots.
Benefits of Nattokinase on Blood Clotting and Fibrolysis
Nattokinase is regarded as a secure, effective and natural supplement for the treatment of heart and cardiovascular disease with low cost(Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Nagata C, Wada K, Tamura T, Konishi K, Goto Y, Koda S, Kawachi T, Tsuji M, Nakamura K; Am J Clin Nutr. 2017 Feb; 105(2):426-431.). Both the animal(Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Sumi H, Hamada H, Nakanishi K, Hiratani H; Acta Haematol. 1990; 84(3):139-43.) and human studies(A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles.
Kurosawa Y, Nirengi S, Homma T, Esaki K, Ohta M, Clark JF, Hamaoka T; Sci Rep. 2015 Jun 25; 5():11601.) have shown that nattokinase could provide protection to the circulatory system by thinning the blood and dissolving blood clots. In a dogs study, thrombi that chemically induced in the major leg vein were dissolved completely within 5 hours and blood flood was restored to normal level(Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Sumi H, Hamada H, Nakanishi K, Hiratani H; Acta Haematol. 1990; 84(3):139-43.) when dogs were orally administered four Nattokinase capsules (2000 FU/capsule). And in a rat study, rats restored the arterial blood flow by 62% after treated by nattokinase; which demonstrated that nattokinase have a strong thrombolytic effect(Transport of nattokinase across the rat intestinal tract. Fujita M, Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, Nishimuro S; Biol Pharm Bull. 1995 Sep; 8(9):1194-6.).
Jang’ s study in 2013(Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation.Jang JY, Kim TS, Cai J, Kim J, Kim Y, Shin K, Kim KS, Park SK, Lee SP, Choi EK, Rhee MH, Kim YB; Lab Anim Res. 2013 Dec; 29(4):221-5.) showed that nattokinase have an effect on oxidative injury-mediated arterial thrombosis. Nattokinase can obviously inhibit the thrombus formation and platelet aggregation caused by giving the ferric chloride (FeCl3) to the injured arteries. This effect of nattokinase is similar to aspirin which is as a regular blood thinner, however nattokinase won’t bring any side effects like bleeding and gastric ulcers as aspirin does. Nattokinase was also reported to treat the inflammation-induced venal thrombosis. The researchers use κ-Carrageenan-induced inflammatory thrombi formation in rat tails to test the pharmacological effect of nattokinase. By gavage administration of nattokinase to rats, higher levels of fibrin degradation product (FDP) fragments and d-dimers were detected in blood samples 12 hours later. By using biopsy analysis, there was more than 50% decrease in thrombosis was found in blood vessels of the rat tail(Thrombolytic effects in vivo of nattokinase in a carrageenan-induced rat model of thrombosis. Xu J, Du M, Yang X, Chen Q, Chen H, Lin DH; Acta Haematol. 2014; 132(2):247-53.).
Unlike most proteins, nattokinase can resist strong acidic gastric fluids in human stomach and can reach to the later section of human digestive tract. A study in 1995 showed that nattokinase could keep a complete form and be assimilated from the intestinal tract of rats(Transport of nattokinase across the rat intestinal tract. Fujita M, Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, Nishimuro S; Biol Pharm Bull. 1995 Sep; 18(9):1194-6.). Later in 2003, an USA research team also found that nattokinase could keep its intact form by observing the serum of healthy human subjects who are orally give nattokinase(A pilot study on the serum pharmacokinetics of nattokinase in humans following a single, oral, daily dose. Ero MP, Ng CM, Mihailovski T, Harvey NR, Lewis BH; Altern Ther Health Med. 2013 May-Jun; 19(3):16-9.). The reason why nattokinase can remain intact in gastrointestinal tract is because it can resist high temperature at 50°C as well as pH value at 10(Nattokinase: production and application. Dabbagh F, Negahdaripour M, Berenjian A, Behfar A, Mohammadi F, Zamani M, Irajie C, Ghasemi Y; Appl Microbiol Biotechnol. 2014 Nov; 98(22):9199-206.). In recent years, there are many finished nattokinase products sold in Asia, EU and North America and it has been widely applied as a natural supplement for thinning blood, preventing blood clots and improving blood circulation. Other benefits of nattokinase may carry include treatment of hypertension, stroke, Alzheimer’s disease, and atherosclerosis.
Recommended Dosage
There is not enough evidence to suggest the optimal dose of oral nattokinase, but studies in humans(Peng Y, Yang X, Zhang Y. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol . 2005;69(2):126-132.) tend to use around 100 mg (equivalent to 2,000 fibrinolytic units) taken up to 3 times a day.
Production Flow-chart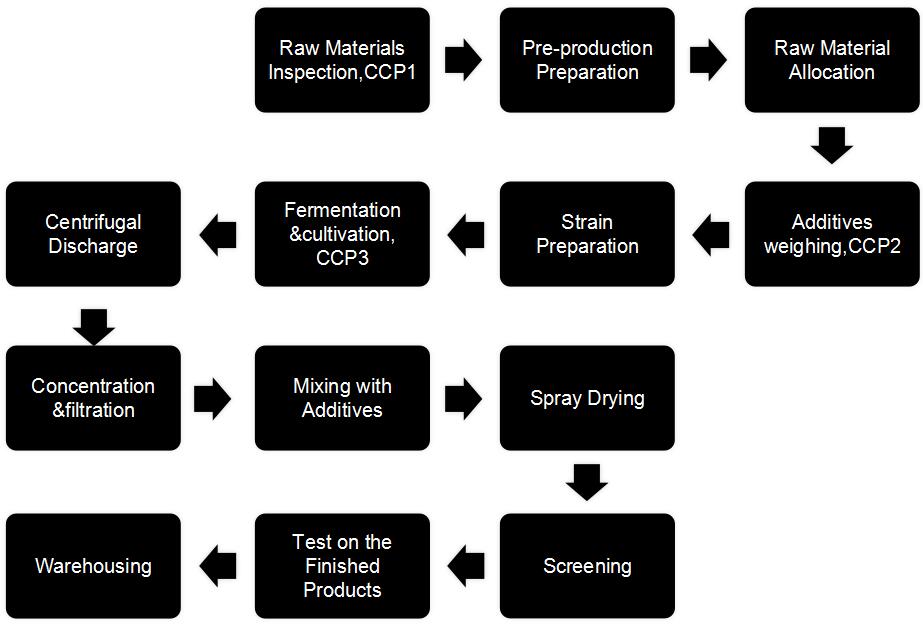 1. Raw Materials Inspection(the critical control point as CCP1)
1. Raw Materials Inspection(the critical control point as CCP1)
Lab personnel conduct random inspection on raw materials with reference to company’s codes and standards for raw materials inspection.
1.1, Raw materials packages should be in good condition with no such phenomenon as dampness and squeeze.
1.2, Raw materials should be from qualified suppliers with Production Certificate and the suppliers can provide with in-house test report, the third report and legal sales contract.
1.3, Appearance, moisture, physical&chemical, and microbe inspection should be conformed to company’s standard.
2. Pre-production Preparation
Production department should check on the water, electricity and gas supply and clean&sterilize the equipment before production.
2.1, Warehouse staff need to check on the inventory of the raw materials and Purchasing Department need to make a timely purchase based on warehouse feedback.
2.2, Monitor on the strain condition.
2.3, Check on the equipment condition, clean the valves&pipes, sterilize all the equipment; clean&dry the fermentation tank, calibrate the dissolved oxygen probe, prepare defoaming agents and clean the spray drying tower.
3.Raw Material Allocation
Allocate the raw materials and accessory materials based on production order.
3.1, Warehouse staff make the material requisition form and confirm the batch number, name, specification, validity period and quantity of raw materials for production.
3.2, With reference to material requisition form, materials handling staff and picking staff prepare the raw materials and ensure the quantity is conformed.
3.3, Materials picking staff should check on the condition of raw materials and ensure there is no such phenomenon as dampness and mildew.
4.Additives weighing(The critical control point as CCP2)
Weigh the additives by using 0.01 gram precision electronic scale and stored in specified packages for next step.
4.1, Warehouse staff make the material requisition form and confirm the batch number, name, specification, validity period and quantity of additive materials for production.
4.2, With reference to material requisition form, materials handling staff and picking staff prepare the raw materials and ensure the quantity is conformed.
4.3, Materials picking staff should check on the condition of raw materials and ensure there is no such phenomenon as dampness and mildew.
5.Strain Preparation
Cultivate the bacillus subtilis var natto strain.
6.Fermentation and cultivation(The critical control point as CCP2)
Cultivate in the fermentation tank and continuously monitor the product’s enzymatic activity by adjusting the dissolved oxygen and temperature under strict disinfection control.
6.1, Conduct empty tank sterilization and filled tank sterilization.
6.2, Put the raw materials in fermentation tank and sterilize the materials at a constant temperature(121±2℃) for 30 minutes.
6.3, Inoculate the seed solution of the small fermentation tank to a large fermentation tank with the pre-confirmation of the lab.
6.4, Manually control the air admission and cooling when fermentation begins, the upper limit of temperature alarm is at 38℃ and the lower at 36.5 degrees. Air exhaust and rotation rate are decided by fermentation time(20-36 hours).
6.5, Sample-grabber should fill out the Sampling Process Monitoring Form and take samples at the 12th hour and every 4 hours after that. PH value should be controlled between 6.0-7.0 for sampling.
6.6, Steam sterilize the discharge port for 20 minutes and start the discharge valve after it cools off.
6.7, Clean all articles that relate to this batch of production and manually dry the fermentation tank by heating at 50℃for 20 minutes.
7.Centrifugate the discharged zymotic fluid
Discharged zymotic fluid are separated for two times to get rid of waste residue.
7.1, The temperature of fermentation tank will decrease to 25℃ after the 36 hours’ fermentation.
7.2, Centifugate the discharged zymotic fluid which is 6 ton for one tank.
7.3, Control the fluid flow at 0.8-1 ton
7.4, The temperature of centrifugal machine should be not higher than 37℃.
7.5, Rotation rate and intensity of centrifugal machine are 6598r/min and 1.6mm/s respectively.
8.Filtration& concentration
Filtrate and concentrate the centrifuged fluids for higher concentration.
8.1, Filtrate by ceramic membrane
8.1.1, Amount of centrifuged supernatant fluids is about 3.5-3.8 ton and the processing quantity is 2ton/hour.
8.1.2, Circulate the cooling water unit to control the temperature of centrifuged supernatant tank and ceramic membrane supernatant tank at 15-20℃.
8.1.3, Control the temperature of ceramic membrane below 37℃.
8.1.4, Sampling test at every one hour; control the PH value at 6.5-7.0.
8.2, Concentrate and filtrate by organic membrane.
8.2.1, Amount of ceramic membrane supernatant fluids is about 3 ton.
8.2.2, Shear fore of feed liquid pump keeps at zero and works at the speed of 30m³/h.
8.2.3, Concentrate the organic membrane liquid at 4-fold to about 1.5 ton.
8.2.4, Control the temperature of ceramic membrane below 15-20℃.
8.2.5, Sampling test at every one hour; control the PH value at 6.5-7.0.
9.Mixing with Additives
Workers add maltodextrin and skimmed milk powder into mixing tank and stir evenly.
9.1, With reference to formulation, mixing is operated in class 100,000 clean room.
9.2, Start the bottom stirring device in case that the pipes are blocked.
10.Spray Drying
Spray drying the concentrated extractum to semi-finished product
10.1, The inlet temperature is controlled at 160 C, and the outlet temperature is 65 C.
10.2, The discharge pump frequency is controlled at around 90.
10.3, The drying tower is heated for half an hour before it can be fed.
10.4, Observe whether the discharged powder is dried.
11. Screening
Screening the dried natto kinase powder and ensure the particle size reach to 100 mesh.
11.1, Screening is operated in class 100,000 clean room.
11.2, Pass through 100 mesh size screen.
12.Test on the Finished Products
The laboratory completes the sampling and inspection according to the enterprise standard; QC should review the entire production control process and ensure every step is conformed.
13. Warehousing
Pack the finished kinase powder with 2 layers food-grade plastic bags in 25kg/fiber drum and label with the accurate specification and batch information before the products are put in storage.
13.1, Check whether the packing materials are clean with no contamination.
13.2, Check whether the label information is correct,including batch number, specification, quantity, and amount.
13.3, Put into storage. The the outside packing box labels should conform to the enterprise standard.
14.Report and record the production information.




