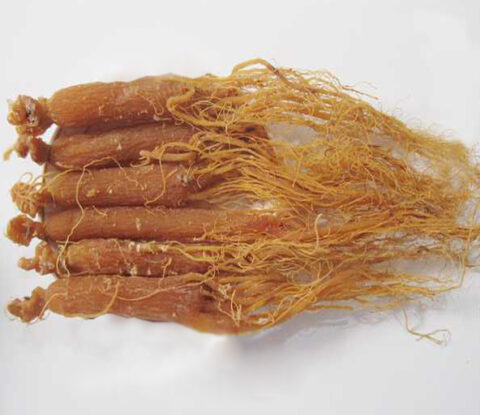
Red Ginseng Extract Powder 10:1, 20:1, 50:1 TLC
The core efficacy of red ginseng is to greatly replenish qi, restore pulse and strengthen the body, and nourish qi and blood. Modern research has shown that it has significant anti fatigue, immune enhancement, cognitive and memory improvement effects, as well as the ability to regulate blood pressure and blood sugar, protect the cardiovascular system, and possess antioxidant and anti-aging activities. It is suitable for body weakness, fatigue, and post disease rehabilitation regulation
Red Ginseng Extract Powder 10:1, 20:1, 50:1 TLC
【Botanical source】: Panax ginseng C. A. Mey.
【Part used】: Root
【Specification】: 10:1, 20:1, 50:1 TLC
【Extraction solvents】: Water
【Appearance】: Brownish fine powder
【Particle size】: 95% pass 80 mesh size
【Main ingredients】: Red ginseng is steamed and processed ginseng, with the core active ingredient being ginsenosides. Its types (such as Rg1, Rb1, Re, Rg3) and contents change after processing. In addition, it contains polysaccharides, peptides, maltol, acidic polysaccharides, and various trace elements. These ingredients collectively endow red ginseng with the effects of nourishing and strengthening the body, anti fatigue, regulating immunity, improving cardiovascular function, and antioxidation.
【Storage conditions】:Store at room temperature in a sealed manner, away from light, and in a ventilated, cool, and dry environment.
【Shelf life】: 24 months from the production date

Red Ginseng Extract Powder Production Flowchart
Red Ginseng raw materials -Coarse powder(40 mesh) -Low temperature water extraction – 1st Reflux Extraction(10 times water,2 Hrs) – 2nd Reflux Extraction8 times water,1.5 Hrs) – 3rd Reflux Extraction(6 times water,1 Hrs) – Extraction Solution-combine&Filtrate-Concentrate-Extractum-spray drying – screening – packaging – detection of physical and chemical indicators – warehousing
Specification Sheet of Red Ginseng Extract Powder
| Product name: | Red Ginseng Extract | ||
| Specification: | 10:1 TLC | ||
| Part used: | Root of Panax ginseng C. A. Mey. | ||
| Solvent used: | Water | ||
| Process: | Raw materials crushed, extracted, concentrated and spray-dried to powder | ||
| Non GMO according to regulation (EC) 1829/2003 and 1830/2003 or United States requirements. Non allergen according to Directive 2007/68 amending Annex IIIa to Directive 2000/13/EC and US Food allergen labelling and consumer protection act 2004. | |||
| Heavy Metals: | |||
| Lead: | NMT 3ppm | Cadmium: | NMT 1ppm |
| Arsenic: | NMT 2ppm | Mercury: | NMT 1ppm |
| Residual solvents: | Comply to USP | ||
| Pesticides residues: | Conform to Regulation USP<561> | ||
| Microbiology: | |||
| Total plate count: | 10000cfu/g Max | Yeasts and molds: | 1000cfu/g Max |
| E.coli: | Not detected in (g)10 | Salmonella spp.: | Not detected in (g)25 |
| Staphylococcus aureus: | Not detected in (g)10 | Clostridium spp.: | Not Present in 0.1 g of food |
| Organoleptic quality | Method | Specifications | |
| Aspect: | Visual : ( CQ-MO-148) | Powder | |
| Color: | Visual : ( CQ-MO-148) | Brownish | |
| Flavor: | Sensory: (CQ-MO-148) | Characteristic | |
| Analytical quality | Method | Specifications | |
| Identification: | TLC | Conform | |
| Loss on drying: | USP <731> | < 10% | |
| Bulk density: | USP <616> Method I | 40 – 60 g/100mL | |
| Particle size: | Analytical sieving || USP <786> | 100% through 80meshes | |
| Packaging suitable for foodstuff. | |||
Extended Reading
Modern Research on Red Ginseng Extract
Chemical Components
Red Ginseng (Panax ginseng C.A. Meyer, steamed and dried) exhibits a unique chemical profile due to the heat-processing which converts malonyl-ginsenosides to neutral ginsenosides and generates novel compounds:
- Ginsenosides (Primary Bioactives): Over 40 identified. Processing increases rare, bioactive ginsenosides:
- Protopanaxadiol (PPD) Group: Rb₁, Rb₂, Rc, Rd, Rg₃, Rh₂, Compound K (metabolite). Rg₃ and Rh₂ are signature “rare” ginsenosides significantly increased in red ginseng.
- Protopanaxatriol (PPT) Group: Rg₁, Re, Rf, Rg₂, Rh₁.
- Oleanane Group: Ro.
- Polyacetylenes: Panaxynol, panaxydol (exhibit cytotoxic activity).
- Polysaccharides: Acidic polysaccharides (ginsenan) with immunomodulatory effects.
- Peptides & Amino Acids: Specific bioactive peptides (e.g., ginseng oligopeptides) with antioxidant properties.
- Melanoidins: Maillard reaction products formed during steaming, contributing to antioxidant activity and characteristic color.
- Other: Phenolic compounds, vitamins, and minerals.
Health Benefits (Evidence-Based)
- Adaptogenic & Anti-Fatigue Effects
- Mechanism: Modulates the HPA axis, reduces cortisol levels, and enhances mitochondrial function (↑ATP production). Ginsenosides Rb₁, Rg₁, and compound K are key players.
- Clinical Evidence: Multiple RCTs and meta-analyses confirm significant reductions in physical and mental fatigue scores (e.g., in chronic fatigue patients and athletes), and improvements in quality of life.
- Cognitive Function & Neuroprotection
- Mechanism: Promotes neurite outgrowth, inhibits Aβ aggregation and tau phosphorylation, modulates neurotransmitters (ACh, DA, 5-HT), and exhibits anti-neuroinflammation via NF-κB and MAPK pathways.
- Clinical Evidence: Systematic reviews show modest improvements in cognitive performance (memory, attention) in both healthy adults and those with mild cognitive impairment.
- Cardiovascular Health
- Endothelial Function: Improves flow-mediated dilation (FMD) by stimulating eNOS-derived NO production.
- Blood Pressure: Exhibits bidirectional regulation—mild lowering effect in hypertension, potentially via modulation of endothelial function and the RAAS system.
- Anti-platelet: Ginsenoside Rg₃ inhibits platelet aggregation.
- Immunomodulation & Anticancer Adjuvant
- Immunity: Enhances NK cell activity, macrophage phagocytosis, and vaccine efficacy (e.g., influenza) via polysaccharides and ginsenosides.
- Cancer: Primarily studied as an adjuvant. Improves cancer-related fatigue, quality of life during chemotherapy, and may reduce some side effects. In vitro studies show Rg₃ and Rh₂ induce apoptosis and inhibit metastasis (anti-angiogenesis, MMP inhibition).
- Metabolic Health
- Glycemic Control: Improves insulin sensitivity and reduces fasting blood glucose via AMPK activation and PPAR-γ modulation.
- Erectile Dysfunction: Enhances NO-mediated penile blood flow; several RCTs show improvement in IIEF scores.
- Skin Health & Anti-Aging
- Topical and oral administration improve skin hydration, elasticity, and wrinkles by promoting collagen synthesis and inhibiting MMP-1. Antioxidant effects protect against photoaging.
Interactions
- Anticoagulant/Antiplatelet Drugs (Warfarin, Aspirin, Clopidogrel): MAJOR CONCERN. Case reports of INR elevation and bleeding with warfarin. Ginsenosides can inhibit platelet aggregation and may potentiate effects. Close monitoring is essential.
- Immunosuppressants (Cyclosporine, Tacrolimus): May counteract intended immunosuppression in transplant patients. Contraindicated.
- Stimulants (Caffeine, MAOIs): Additive stimulant effects may cause insomnia, nervousness, or hypertension.
- Antidiabetic Drugs (Insulin, Metformin): Potentially additive hypoglycemic effect; monitor blood glucose.
- Cytochrome P450 Enzymes: In vitro data is complex and ginsenoside-specific (some inhibit CYP3A4, others induce). Clinical significance for drug metabolism is uncertain but warrants caution with narrow-therapeutic-index drugs.
Taboos & Warnings
- Pregnancy & Lactation: Avoid due to potential hormonal and stimulant effects. Insufficient safety data.
- Hormone-Sensitive Cancers (Breast, Ovarian, Endometrial, Prostate): Theoretical risk due to weak estrogenic activity of some ginsenosides in vitro; clinical relevance debated but caution advised.
- Autoimmune Diseases (RA, Lupus, MS): May stimulate immune activity, potentially exacerbating conditions.
- Acute Illness: Traditional TCM contraindication for use during acute infections, fever, or “excess” patterns.
- Insomnia & Anxiety: May exacerbate symptoms in sensitive individuals due to its “Qi-invigorating” (stimulant) properties.
- Hypertension: While often used for regulation, high doses may cause or worsen hypertension in some individuals (“ginseng abuse syndrome”).
- Surgery: Discontinue at least 7-14 days prior due to bleeding and cardiovascular interaction risks.
Applications
- Nutraceuticals & Dietary Supplements: Capsules, tablets, liquid extracts, and powders for energy, immunity, and cognitive support.
- Functional Foods & Beverages: Energy drinks, fortified teas, snacks, and confectionery.
- Cosmeceuticals: Anti-aging serums, moisturizers, and masks for improved skin texture and barrier function.
- Pharmaceutical Adjuvants: Investigated as adjuncts in chemotherapy, radiotherapy, and for managing cancer-related fatigue.
- Clinical Practice: Used in integrative medicine for fatigue recovery, convalescence, and geriatric support.
References
- Kim, H. J., et al. (2021). Red ginseng and its major ginsenosides for the treatment of erectile dysfunction: A systematic review. The World Journal of Men’s Health, 39(3), 466-478.
- Lee, S. M., et al. (2020). Red ginseng for fatigue: A systematic review of randomized controlled trials. Nutrients, 12(5), 1410. (Key meta-analysis on fatigue)
- Ratan, Z. A., et al. (2021). Pharmacological potential of ginseng and its major component ginsenosides. Journal of Ginseng Research, 45(2), 199-210. (Comprehensive review of mechanisms)
- Choi, J., et al. (2013). Influence of heat processing on the chemical constituents and antitumor activity of red ginseng. Journal of Ginseng Research, 37(3), 257-267. (Chemistry changes with processing)
- Jin, Y., et al. (2019). Anti-diabetic effects of red ginseng via modulation of gut microbiota and metabolic pathways. Journal of Ginseng Research, 43(4), 580-589. (Modern microbiome research)
- Kim, J. H., et al. (2018). Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. Journal of Ginseng Research, 42(3), 264-269.
- Lee, C. H., & Kim, J. H. (2014). A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research, 38(3), 161-166.
- Shergis, J. L., et al. (2019). Therapeutic potential of Panax ginseng and ginsenosides in cardiovascular disease. Pharmacological Research, 141, 122-132. (Clinical evidence focus)
- Zhu, J., et al. (2022). Red ginseng for cognitive function: An overview of systematic reviews. Frontiers in Pharmacology, 13, 870098.
- Qi, L. W., et al. (2011). Metabolism of ginsenosides and the intestinal microbiota. Planta Medica, 77(16), 1728-1731. (Key paper on bioavailability and gut microbiota role)
- Clinical Interaction: Lee, S. H., et al. (2008). Interaction between warfarin and Panax ginseng in ischemic stroke patients. Journal of Alternative and Complementary Medicine, 14(6), 715-721. (Important interaction case study)
- FDA GRAS Notice: GRN No. 659 (2016).
Note: This summary is for informational purposes. It may interact with medications and is contraindicated in certain conditions. Consult a healthcare professional before therapeutic use, particularly regarding its estrogenic activity.